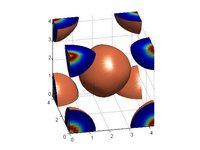
Hi Everyone,
I am calculating the band structure of Cu2O. I use the input variables, acell, spgroup, xred to define the unit cell of Cu2O. Everything is fine. However, when I draw the density figure, Cu atom is lost. In the output file, six atoms are all present. It is in agreement with the Cu2O structure.
Could anybody help me to find the reason. please see the attached jpg images. here is the input file.
#Definition of the unit cell
acell 3*4.261 angstrom #
angdeg 3*90
spgroup 224
brvltt 1
spgaxor 1
ntypat 2 #
znucl 8 29 # O and Cu
natom 6 #
natrd 2
typat 1 2 #
xred
0.00 0.000 0.000
0.25 0.25 0.25
...
output file
xred 0.0000000000E+00 0.0000000000E+00 0.0000000000E+00
2.5000000000E-01 2.5000000000E-01 2.5000000000E-01
7.5000000000E-01 7.5000000000E-01 2.5000000000E-01
7.5000000000E-01 2.5000000000E-01 7.5000000000E-01
5.0000000000E-01 5.0000000000E-01 5.0000000000E-01
2.5000000000E-01 7.5000000000E-01 7.5000000000E-01
Density loses one atom?
Moderator: bguster
Re: Density loses one atom?
What you see is normal: the oxygens have taken all the valence electrons, and none are left on the Cu. The atom is there, but bare. You might have to consider a pseudopotential with semicore states for Cu, as they are exposed and may be un-atomic-like in Cu2O... Then you should see something on Cu sites as well...
Matthieu
Matthieu
Matthieu Verstraete
University of Liege, Belgium
University of Liege, Belgium
Re: Density loses one atom?
Matthieu,
Thanks, I got it.
SteveZ
Thanks, I got it.
SteveZ
mverstra wrote:What you see is normal: the oxygens have taken all the valence electrons, and none are left on the Cu. The atom is there, but bare. You might have to consider a pseudopotential with semicore states for Cu, as they are exposed and may be un-atomic-like in Cu2O... Then you should see something on Cu sites as well...
Matthieu